The split vote means the vaccine schedule may be altered again soon.
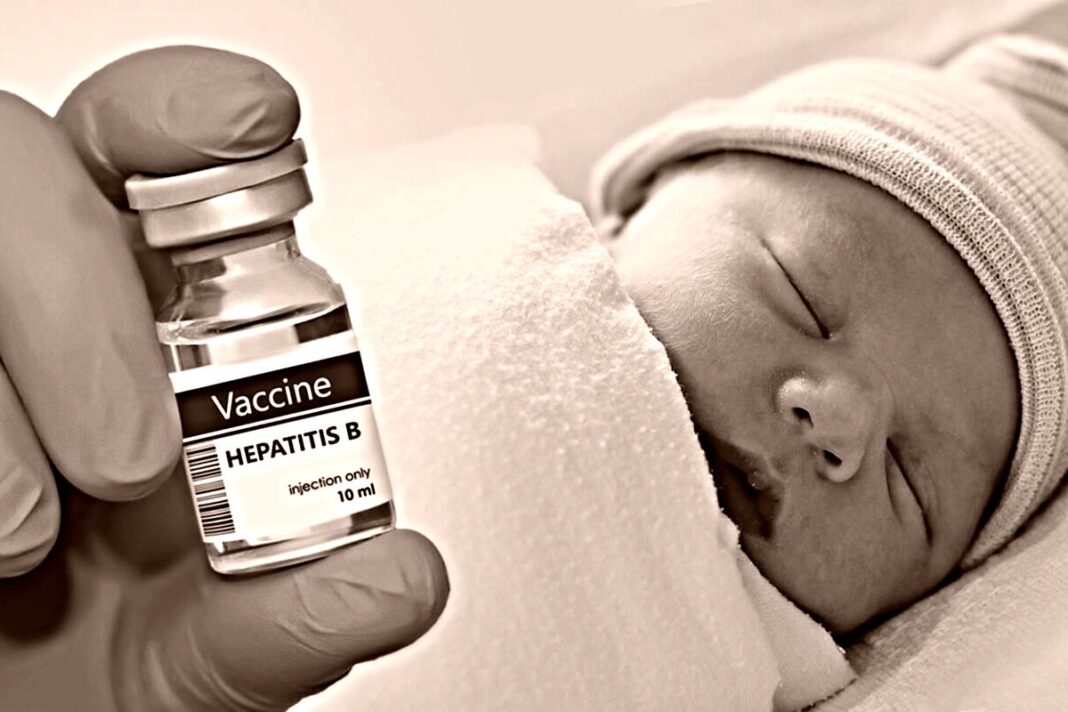
A panel of experts selected by Health Secretary Robert F. Kennedy Jr. on Dec. 5 voted to recommend federal officials stop directing parents to get their infants quickly vaccinated against hepatitis B, unless the babies’ mothers test positive for the virus.
The Centers for Disease Control and Prevention currently recommends virtually all newborns receive a hepatitis B vaccine within 24 hours of birth. The CDC should, moving forward, no longer recommend that infants born to women who test negative for hepatitis B receive a vaccine against the virus, unless parents and health care providers decide to have the infants vaccinated following consideration of the risks and benefits of vaccination, according to a majority of the Advisory Committee on Immunization Practices (ACIP).
Members who voted in favor said safety and effectiveness data for the current recommendations are inadequate.
“What we are trying to here is to go back to basic, good public health policy that is based on risk, that is based on informed consent and individual decision-making, when parents can think about the assessment of the risk and the benefits and make a decision,” Retsef Levi, one of the members, said ahead of the vote.
The CDC’s director typically adopts ACIP’s advice without alteration. Jim O’Neill, who is also Kennedy’s deputy secretary, is serving as acting CDC director.
The Department of Health and Human Services (HHS) declined to comment. The CDC did not respond to a query by publication time.
Hepatitis B
Hepatitis B is a virus that causes problems such as dark urine and liver infection. It can be transmitted via exposure to bodily fluids from an infected person, as well as from a mother to child during pregnancy.
About 0.5 percent of pregnant women test positive for hepatitis B, and in 2021, 17,827 children were born to mothers who tested positive, according to an estimate from the National Center for Health Statistics. While about eight in 10 births overall that year were to U.S.-born mothers, a majority of mothers who tested positive for hepatitis B were not born in the United States.
Roughly 80 percent of children born in the United States in 2020 and 2021 received a hepatitis B vaccine dose within three days of birth, according to the CDC.
Officials have since 1991 recommended that all infants receive a hepatitis B vaccine shortly after birth. The CDC and some experts outside the government credit the vaccine with being behind a sharp decline in acute cases of hepatitis B.
“This disease has gone down in the United States thanks to the effectiveness of our current immunization program,” Dr. Cody Meissner, one of the ACIP members who voted not to change the current schedule, said on Thursday during the two-day meeting.
But the level of antibodies, one measure of effectiveness, in vaccinated people drops over time, and has been detected to be at low levels at older ages, particularly among children who received a shot early in life, Cynthia Nevison, a CDC contractor, told panel members. She cited several papers, including a 2016 study of adults and children in Alaska.
The CDC acknowledges on its website that antibodies in vaccinated persons wane over time but also says that immune memory holds up.
Nevison also noted that hepatitis B cases were already declining from a peak in 1985, when the universal birth dose was first introduced, contrary to a 1991 CDC publication that outlined why the hepatitis B campaign was being expanded to all newborns and stated that “selective vaccination of persons with identified risk factors … has not lowered the incidence of hepatitis B.”