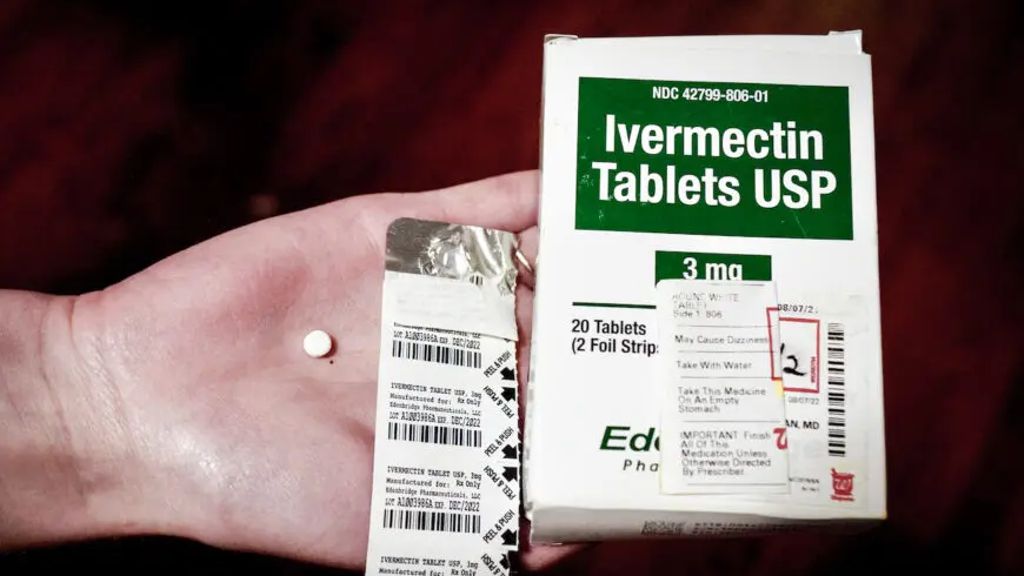
Despite the proven efficacy of ivermectin in treating COVID-19, some studies in top-tier journals have concluded otherwise. Which data should we trust?
Health Viewpoints
The use of ivermectin to treat COVID-19 is an ongoing debate. The central conflict is that while many doctors have reported success in using ivermectin, some studies published in major journals suggest it is in fact ineffective.
Even as the FDA recently has been removing misinformation it posted about ivermectin, the agency has maintained its original position regarding its effectiveness, namely that there isn’t evidence.
People who trust ivermectin claim the studies showing ineffectiveness are fraudulent, while people who are skeptical of its use for treating COVID-19 view it as an anti-science conspiracy theory.
As a professional with decades of research experience conducting dozens of clinical trials on antiviral drugs, I decided to dive deep into the studies purporting ivermectin’s ineffectiveness. What I found shocked me.
Legacy Media Report Ineffectiveness
Numerous preclinical studies have found that ivermectin has a broad range of effects on COVID-19, spanning from its initial impact on viral infection to the pathological changes the virus causes in our bodies.
Ivermectin inhibits the entire life cycle of SARS-CoV-2 in our cells from attachment, spreading, and replication (1, 2, 3).
Moreover, ivermectin is anti-inflammatory and organ-protective, which can potentially protect against severe COVID-related lung damage and acute respiratory distress syndrome, heart-relatedcomplications, and blood clots.
Ivermectin exceeds the approved antiviral effects of other medications, including Paxlovid, molnupiravir and remdesivir, which only target the virus and lack anti-inflammatory and organ-protective effects. Monoclonal antibodies have to be constructed specific to each variant and are very expensive.
In the pharmaceutical industry, clinical trials are commonly used to evaluate the efficacy and safety of drugs once their mechanism is demonstrated. There are two types of clinical trials: observational and interventional.
Observational studies are often conducted by doctors in clinical, hospital, or community settings to analyze the effects of drugs. The data is collected as observed in clinical practice with minimal interference.
By Yuhong Dong